Dealing with dry rot – insights from fungal biology
The dry rot fungus Serpula (formerly Merulius) lacrymans is well known for destroying timbers in buildings and spreading from floor to floor through hidden spaces. Its mysterious appearances throughout an affected building have led to a sometimes almost mystical fear of its destructive abilities, as though it is something like a biblical plague. However, S.lacrymans (or dry rot) is only a woodland fungus that happens to have developed a capacity to grow in buildings. Its ancestral habitat is cool pine forests, where it feeds on fallen dead wood on the moist forest floor.
A building containing softwood embedded in damp masonry provides all the conditions to which the fungus has become adapted by natural selection in its original forest home: damp softwood to feed on, a moist surface from which it can scavenge mineral nutrients, and damp enclosed spaces to grow in. By understanding the fungus’s biological requirements, evolved in its natural habitat, we can alter the conditions within the building to make them unsuitable for its growth.
S.lacrymans occurs in genetically distinct races in different parts of the world. Each race probably originated when a strain of S.lacrymans growing in dead wood on a pine forest floor made the jump to conifer wood in buildings. Populations of the fungus are made up of individuals, with each individual growing network the mycelium made of microscopic filaments, the hyphae. Individuals that meet can fuse hyphae to join into a single network.
Identifying S.lacrymans (Dry Rot) in buildings
The sponge-like sporophores (fruiting bodies) of S.lacrymans are often produced in ceiling cornices or corners of walls, oriented so that the pores are vertical, allowing spores to drop out into the air. Sporophores produce millions of rusty red spores a day which collect as a red dust on horizontal surfaces in unventilated rooms.
When sporophores are absent S.lacrymans can be identified by visual inspection and if necessary analysis of decayed wood. S.lacrymans affects softwood. Early tensile strength loss occurs in wood that may still appear intact because S.lacrymans enzymes can extract sugars from cellulose without at first disturbing the lignin framework of the wood cells.
Genomic analysis recently showed that S. lacrymans has a highly evolved and sophisticated system of scores of cellulose digesting enzymes which it synthesises whenever its hyphae meet a wood food source. Eventually, as cellulose is progressively removed, the wood becomes brown, breaks up by transverse and horizontal cracks into cubes, and eventually collapses into a dry brown powdery lignin residue.
Painted surfaces of timber such as skirting boards are usually colonised by mycelium growing through damp underlying masonry. The paint skin is easily dented with a chisel where the timber beneath has been decayed.
Mycelial cords − aggregates of hyphae visible to the naked eye − are produced by both S. lacrymans, and its close relative Coniophora puteana. These Agaricomycete brown rotting species are the most common cause of timber decay in northern European buildings. Mycelial cords are strands of fungus from one to several millimetres wide, white in colour changing to yellow and brown. Cords usually remain, dried and brown, after an outbreak has died down. They are often revealed when water-impermeable coverings such as oil-based paint, vinyl flooring and rubber-backed carpet are removed.
DNA testing can identify traces of live or even dead fungal tissue. Where the expense is warranted, samples may be sent to specialist laboratories. Unique volatiles of active dry rot can be detected by trained dogs.
Coniophora puteana can be confused with S.lacrymans because it has cords and a similar type of rot, but it does not spread as far and is usually limited to damper areas, hence the name cellar fungus.
It spreads through buildings by mycelial growth
S.lacrymans can spread through buildings because of its ability to extend for distances of several metres from a wood food base over non-nutrient surfaces. In nature, this allows it to capture scattered pieces of dead wood by growing across the forest floor.
In buildings, mycelium growing from infected wood grows outwards over walls and behind plaster and flooring materials. The microscopic hyphae and fine mycelial cords are invisible to the naked eye and grow through spaces as small as a few microns (hundredths of a mm) wide, until it finds a fresh food source – damp wood or other cellulosic material.
The fungus advances through a building as a leading edge of parallel separate branching hyphae. These are the feeding part of the fungus. They exude enzymes and also scavenge nutrients: carbohydrates from wood, and compounds of mineral nutrients nitrogen, phosphorus and potassium from underlying surfaces.
The microscopic hyphae that initially colonise wood can only grow in wood with a water content of over 20%. There is no evidence that dry wood, with moisture level below 20%, can be colonised through import of water through cords, although mycelium can exude drops, and enclosed spaces round growing mycelium can retain damp.
Nutrients accumulate inside the fungus as sugars and amino acids. They are translocated responsively in the network according to local supply and demand, through mycelial cords that develop around the hyphae connecting old and new colonised wood. Cords carry nutrients in either direction and can move different nutrients in opposite directions.
Starvation for nitrogen, provided the fungus is established in a wood food base that provides abundant carbohydrate, causes faster extension. Mycelium with plenty of nitrogen extends more slowly and branches more. This growth response can be exploited to limit spread of dry rot in buildings.
Extension stops when mycelium is fed with a local application of a non-metabolised amino acid, α- aminoisobutyric acid, AIB, which is translocated in place of food amino acids. AIB is like junk food. The fungus wrongly perceives a nitrogen glut, and switches its development from extension to localised branching. AIB inhibition acts systemically throughout a connected network. Arrest of extension persists indefinitely.
Spread between buildings by spores
An individual grows from a spore produced by a sporophore. Genetic analysis shows that spores produced in sporophores are the source of new outbreaks of dry rot in buildings. There is no support for the theory that dry rot infection can spread between buildings as tissue fragments in wood or on tools.
Survival of mycelium and vegetative spores within infected buildings
S.lacrymans can only grow in cool conditions. Growth is optimal at 20°c, scanty at 26-27°c and the fungus cannot grow above 28°c. Temperature affects the ability of mycelium to survive dormant in dry wood. It can remain viable for up to 8 years at 7.5°c but only 6 months at 27°c. Spores are more resistant to higher temperatures, but are killed by 32h at 60°c or 1h at 100°c.
Treatment
The first step is to locate both the wood and the source of water. Sporophores can develop at distances of 10m from the wood food source, connected to it by cords. A sporophore means that fungal growth is well established in a substantial wood structure. This may require exposure of underlying wall by removal of plaster and fittings. Established procedures used by accredited contractors are effective in modern buildings. Heritage buildings may require a more customised approach to detect and limit dry rot.
For example, a sporophore appearing between masonry joints in the entrance archway of an uninhabited Scottish castle was traced back to a wooden lintel nobody knew was there, embedded in a wall 15 metres away. The source of damp was condensation from the outer surface of a cold water tank in a tower percolating down through the wall into the lintel. The fungus had crossed a masonry wall in the form of cords connected to the sporophore which was emerging into the nearest available open air.
In a Victorian house in Oxford, massive mycelial growth was found colonising a wood fuel store in a cellar. The fungus originated from the floor above where rising damp caused by a blocked air brick and soil against an external wall. The unventilated space in the cellar stimulated mycelial growth.
A Victorian dining room was panelled with mahogany veneer curved round the inside of a circular turret, secured by softwood battens. Water seeping in through the exterior wall behind from a broken downpipe had allowed S.lacrymans to colonise the damp softwood battens and expand into the space behind the panelling.
In all these cases the treatment was to expose and ventilate the affected parts of the building, remove as much of the affected timber as feasible, and repair sources of water ingress.
Conservative treatment in historic buildings
Where large buildings leaky buildings are uninhabited, as in the case of fire damage or disputed ownership rapid effective remedial treatment may be impossible. AIB application to arrest spread is allowed under a site-by-site HSE experimental license. Electronic damp monitoring is the best safeguard in long-term projects involving neglected buildings.
References
Schmidt, O. (2006). Wood and Tree Fungi: Biology, Damage, Protection and Use. Springer, Belin Heidelberg
Schmidt, O. (2007). Indoor wood-decay basidiomycetes: damage, causal fungi, physiology, identification and characterisation, prevention and control. Mycological Progress 6, 261-279. Lloyd, H. & Singh, J. (2004) Inspection, monitoring and environmental control of timber decay. In:
Building Mycology, ed. Jagjit Singh. E & FN Spon, London. PP. 159-186.
Watkinson, S.C, & Eastwood, D. C. Serpula lacrymans, wood and buildings (2012). In: A.Laskin, Sima Sariaslani and G.Gadd, eds. Advances in Applied Microbiology 78, 121-149.
Sarah Watkinson Consulting
Figures

Sporophore with rusty red spores, which has formed on top of a Jay cloth.

Infected wood showing cubical cracking and exudation of water by the fungus

Infected wood in a painted door frame. The wood has shrunk and broken into cubes behind the paint skin.

Mycelium growing in a wall cavity from softwood battens behind mahogany panelling. Mahogany resistant to colonisation.

Cords of S.lacrymans exposed by removing ceiling plaster. Water ingress from a shrunken lead roof above caused prolonged damp in ceiling battens.
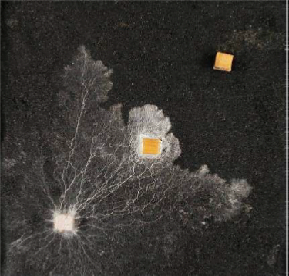
Laboratory experiment demonstrating the chemical 2-aminoisobutyric acid arresting the spread of S.lacrymans. This harmless, but as yet unregistered, substance causes lasting arrest of mycelial extension.